Progesterone attenuates visceral pain through the mucosal mast cell-derived 5-hydroxytryptamine and vagal 5-hydroxytryptamine 3 receptor signaling in rats
Introduction
Sex-dependent differences in the perception and modulation of pain have long been the subject of speculation and investigation[1-3]. Reportedly, women exhibit a higher prevalence of chronic pain syndromes, including irritable bowel syndrome (IBS), temporomandibular disorders, neuropathic pain and fibromyalgia[4, 5]. Moreover, pain severity fluctuates with the menstrual cycle[4, 6]. Estradiol has been proposed to account for the sex-dependent differences observed in females[7, 8]. A link between progesterone and algesic responses has also been reported[9-11]. However, a subgroup of female patients with IBS showed exacerbated abdominal pain during menses[4]. And both clinical and animal studies have shown that high plasma levels of progesterone elevate the pain threshold and attenuate inflammatory hyperalgesia[12-14]. These conflicting findings suggest that the modulation of nociception by progesterone may involve complicated mechanisms.
Sex-dependent differences in the perception and modulation of pain have long been the subject of speculation and investigation[1-3]. Reportedly, women exhibit a higher prevalence of chronic pain syndromes, including irritable bowel syndrome (IBS), temporomandibular disorders, neuropathic pain and fibromyalgia[4, 5]. Moreover, pain severity fluctuates with the menstrual cycle[4, 6]. Estradiol has been proposed to account for the sex-dependent differences observed in females[7, 8]. A link between progesterone and algesic responses has also been reported[9-11]. However, a subgroup of female patients with IBS showed exacerbated abdominal pain during menses[4]. And both clinical and animal studies have shown that high plasma levels of progesterone elevate the pain threshold and attenuate inflammatory hyperalgesia[12-14]. These conflicting findings suggest that the modulation of nociception by progesterone may involve complicated mechanisms.
Therefore, the present study was aimed to determine whether progesterone regulation of visceral pain involves vagal afferents and IMMCs in a rat model of visceral pain. According to our previous studies[23], progesterone was subcutaneously injected to act through both vagal and spinal pathways, or intraduodenally infused to preferentially act through the vagal pathway. We also investigated whether progesterone and estrogen act synergistically in pain modulation.
Material and Methods
Animals
Adult female Sprague-Dawley rats (200-250 g) were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Science (Shanghai, China) and maintained on a normal light-dark cycle, housed singly after surgery and provided with food and water ad libitum. The experimental procedures were performed in accordance with the guidelines for experimental pain in animals published by the International Association for the Study of Pain and were approved by Shanghai Jiaotong University School of Medicine. Most of the animals were ovariectomized (OVX) and tested two weeks after the surgery
Implantation of electrodes and intraluminal catheter
The implantation of electrodes and intraluminal catheter was conducted, as described previously . Briefly, nine days after OVX surgery, with the rats anesthetized, electromyogram (EMG) electrodes were inserted into the abdominal external oblique muscles. The lead wires were then tunneled subcutaneously and exteriorized at the back of the neck. Then, a polyethylene tube was gently inserted into the duodenum (positioned at 2 cm away from the pylorus) from the fundus of the stomach for drug administration. The cannula was fixed with sutures, and its remaining free end was exteriorized at the back of the neck. The rats that were subjected to surgery were allowed to recover for five days.
Perivagal application of capsaicin
Perivagal capsaicin treatment was conducted according to descriptions of the previous studies[23, 24]. Briefly, under anesthesia with sodium pentobarbital, an upper midline laparotomy was performed, and the abdominal vagal nerve trunks were exposed and isolated. Cotton wool soaked in capsaicin (1 mg/ml; Sigma) was placed around the vagal trunks for 30 min to largely block vagal afferent conduction through the induction of desensitization[25, 26]. Vehicle (10% Tween 80 in olive oil) alone was applied to the vagal trunks of the control rats. The rats were used in pain behavioral experiments seven days later
Assessment of visceromotor response
After a fasting period of 18-24 h, the rats were lightly anesthetized. A flexible, 6 cm latex balloon was inserted into the descending colon and rectum such that its end was 1 cm proximal to the anus with a catheter fixed at the tail. Colorectal distension (CRD) was produced by inflating the balloon with air. The pressure was regulated using a distention control device and monitored using a pressure transducer. The visceromotor response (VMR) was evaluated by measuring EMG activity in the external oblique muscles in response to the graded CRD pressure (20, 40 and 60 mmHg respectively). Each CRD consisted of a 20 sec predistension baseline period, a 20 sec distension period and a 20 sec period after the termination of CRD, with a 3 min interstimulus interval.
The EMG signal was collected and analyzed with LabChart (AD Instruments, Bella Vista, NSW, Australia). The raw EMG signals were rectified off[1]line, and the area under the curve (AUC) prior to distention was subtracted from the AUC during the distention.
Behavioral experiments
In the first experiment, we observed the effects of luminal or subcutaneous (sc) administration of progesterone on the VMR produced by CRD. One group of rats received an intraduodenal infusion of progesterone (dissolved in saline, Sigma) at a rate of 0.05 ml/min for 10 min; the dose of progesterone was 3.2 μmol (1 mg), which was similar to that used in previous studies[26]. The control group received vehicle (saline). VMR was measured 30 min later. Another two groups underwent sc injection of saline or progesterone at the same dose 20 min prior to the VMR assessment[23]. To investigate whether the nuclear progesterone receptor (PR) was involved in the effects of luminally applied progesterone, the rats were pretreated with intraperitoneal (ip) injection of the nuclear PR antagonist mifepristone (RU486; 5 mg/ kg), which was dissolved in 0.5% dimethyl sulfoxide (DMSO), 1 h prior to the luminal administration of progesterone[27]. The control group received vehicle pretreatment. To explore the role of vagal afferents, progesterone was infused into rats subjected to pretreatment with perivagal application of capsaicin or vehicle.
The second experiment aimed to examine the roles of 5-HT3Rs and histamine (H1) receptors in progesterone antinociception. Two groups of rats were pretreated with the selective 5-HT3R antagonist granisetron (250 nmol[28], luminally infused, Sigma) or the H1 receptor antagonist pyrilamine (5 mg/kg[29], ip, Sigma) 20 min prior to the luminal administration of progesterone. VMR was tested 30 min after the infusion of progesterone.
The second experiment aimed to examine the roles of 5-HT3Rs and histamine (H1) receptors in progesterone antinociception. Two groups of rats were pretreated with the selective 5-HT3R antagonist granisetron (250 nmol[28], luminally infused, Sigma) or the H1 receptor antagonist pyrilamine (5 mg/kg[29], ip, Sigma) 20 min prior to the luminal administration of progesterone. VMR was tested 30 min after the infusion of progesterone.
In the fourth experiment, the rats were given a luminal administration of progesterone plus estradiol (10 nmol[23], dissolved in 0.5% DMSO; Sigma), or progesterone or estradiol alone. VMR was tested 30 min later.
Duodenal perfusion and measurement of 5-HT content
In the first experiment, duodenal perfusion was performed to observe whether progesterone, when luminally applied or sc injected, can induce 5-HT release into the perfusate. As previously described[23, 31], the rats were anesthetized, the intestine was exposed through an abdominal midline incision, and a polyethylene tube was inserted into the duodenal lumen (positioned 2 cm from the pylorus) from the fundus of stomach. Another tube was inserted at the end of the duodenum next to the ligament of Treitz to allow drainage and perfusate collection. Saline was perfused for 20 min at a rate of 0.2 ml/min to flush the duodenal lumen. Then, in one group of rats, the saline was switched to progesterone (6.4 mM), and perfusion continued at the same speed for 40 min, with the perfusate collected in ice-chilled tubes every 10 min. The control group received a 40 min saline perfusion. Two separate groups were given sc injection of progesterone (1mg) or saline 20 min prior to the perfusion with saline. The 5-HT content in the perfusate was analyzed with an enzyme immunoassay kit according to the manufacturer’s instructions (Beckman Coulter, Fullerton, CA, USA).
In the second experiment, the rats were pretreated with ip injected doxantrazole or vehicle prior to luminal or sc administration of progesterone. Duodenal perfusate was then collected as described above for the determination of 5-HT content.
In the third experiment, the rats were given a luminal administration of progesterone plus estradiol, or progesterone or estradiol alone. Duodenal perfusate was also collected for 5-HT measurement.
Analysis of progesterone, chromogranin A and hista[1]mine concentration
Plasma progesterone concentration after luminal application of progesterone was analyzed with an enzyme immunoassay kit (Cayman Biochemical, Ann Arbor, MI, USA). The chromogranin A content in the serum or duodenal perfusate from progesterone-treated rats was determined using an enzyme immunoassay kit (Adipo Bioscience, Santa Clara, CA, USA). The histamine content in the perfusate was analyzed using an enzyme immunoassay kit (Cayman Biochemical).
c-Fos immunohistochemistry
The rats were given an overdose of sodium pentobarbital (ip) 2 h after the termination of sc or luminally applied progesterone. The rats were then transcardially perfused with fixative containing 4% paraformaldehyde. The left and right nodose ganglia and T6-T10 dorsal root ganglia (DRG) corresponding to the duodenum were removed and fixed in 10% formalin for 24 h. They were then paraffin-embedded and sectioned (4 mm). After epitope retrieval treatment (at 98°C for 15 min in 0.01 M citrate buffer solution, pH 6.0), the sections were incubated with a rabbit anti[1]c-Fos antibody (1: 200; Abcam, HK) overnight at 4°C. The sections were then incubated with a goat anti[1]rabbit horseradish peroxidase-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) for 30 min at room temperature. The c-Fos protein was visualized with a diaminobenzidine (DAB)-enhanced liquid substrate system (Sigma). The number of c-Fos immunoreactive (IR) neurons was calculated in three rats (6 ganglia) per group, with each ganglion counted in 2 non-consecutive sections.
Electron microscopy
Thirty minutes after the sc injection of progesterone, full-thickness duodenal segments were removed and immediately fixed in 2% glutaraldehyde for 2 h at 4°C, then post-fixed in 1% osmic acid. After dehydration in graded alcohols, the blocks were put into epoxy propane and then soaked in a mixed solution of epoxy propane and propylene oxide. The blocks were then embedded and sectioned with an LKB V ultramicrotome (LKB-Produkter AB, Stockholm, Sweden). The sections were stained with lead citrate and observed under an electron microscope (Philips, Eindhoven, Holland) at 80 kV.
Statistical analysis
The data are expressed as the mean ± SEM (standard error of mean). The CRD-evoked VMR was calculated as the AUC per second (area/sec). Statistical analysis was performed using Student’s t-test, one-way analysis of variance (ANOVA) and post hoc by Turkey test, or two-way repeated measures ANOVA followed by the Bonferroni post-test comparisons. Differences of P < 0.05 were considered statistically significant.
Results
Luminally applied progesterone rapidly attenuates the VMR to CRD
We first observed the effect of intraduodenally applied progesterone on CRD-induced VMR in ovariectomized rats. Luminal infusion of vehicle (saline) had no influence on the VMR magnitude [F(1,24) = 0.07, P = 0.80] (Figure 1A), while the application of progesterone (3.2 μmol, equivalent to 1 mg) led to significantly decreased VMR to CRD compared to baseline [F(1,54) = 23.3, P < 0.001] (Figure 1B). The analgesic effect of progesterone occurred at 30 min following luminal infusion and lasted for nearly 1 h (Figure 1C). Luminal infusion of progesterone similarly decreased the VMR in intact female rats (data not shown). The results of the enzyme immunoassay showed that plasma progesterone concentration (<15 ng/ml) in rats treated with luminal progesterone was><15ng/ml) in tats treated with luminal progesterone was not different compared to vehicle-treated rats. In
contrast, sc injection of progesterone at the same dose elevated the EMG levels compared to baseline [F(1,30) = 41.6, P < 0.001] (Figure 1D), which occurred 10 min after drug administration (Figure 2). Moreover, pretreatment with the nuclear PR antagonist RU486 did not influence the antinociceptive effect of progesterone [F(1,24) = 24.4, P < 0.001 vs. baseline] (Figure 1E). RU486 itself had no effect on the VMR (data not shown).
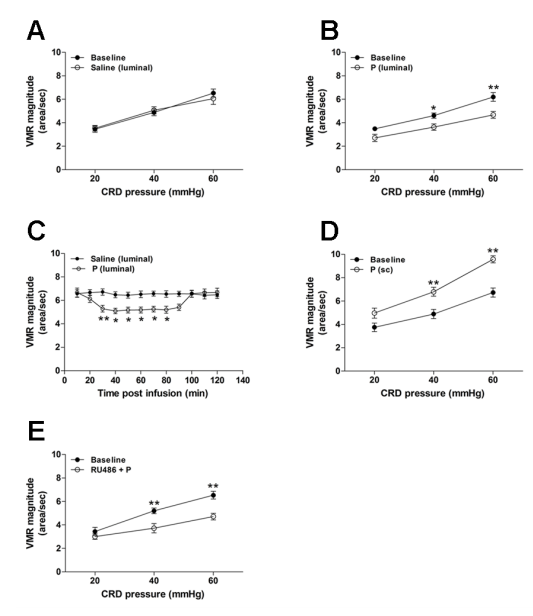
Figure 1. Luminally applied progesterone (P) rapidly attenuates the visceral pain response to CRD in ovariectomized rats. VMR before and after luminal application of saline (vehicle) (A, n = 5) or progesterone (B, n = 10). Progesterone (3.2 μmol) was administered 30 min prior to VMR assessment. Time course of VMR attenuation following luminal (C, n = 6) administration of progesterone. (D) VMR before and after sc injection of progesterone (n = 6). Progesterone (1 mg) was administered 20 min prior to VMR assessment. (E) RU486 pretreatment did not affect the antinociceptive effect of luminally applied progesterone (n = 5). RU486 (5 mg/kg) was ip injected 1 h prior to progesterone. *P < 0.05, **P < 0.001 vs. baseline or saline (two-way repeated measures ANOVA).
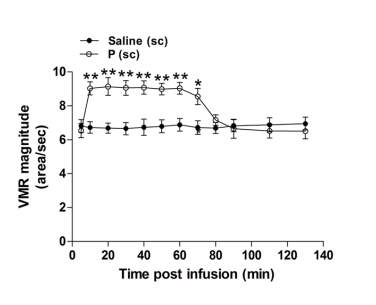
Figure 2. Time course of VMR elevation following sc administration of progesterone (n = 6). *P < 0.05, **P < 0.001 vs. saline (two-way repeated measures ANOVA). In rats treated with luminally or sc administered progesterone, the percentage of c-Fos-IR neurons in the nodose ganglia was significantly increased (luminal: 70.2 ± 6.7 %, vs. vehicle: 24.3 ± 4.3%, P < 0.01; sc: 79.3 ± 6.2%, vs. vehicle: 23.1 ± 4.0%, P < 0.001; n = 6). Only sc injected progesterone led to an increased percentage of c-Fos-IR neurons in the DRG corresponding to the duodenum [luminal: 22.6 ± 4.8%, vs. vehicle:15.8 ± 5.2%, P > 0.05; sc: 70.2 ± 8.8%, vs. vehicle: 29.4 ± 3.9 %, P < 0.001; n = 6] (Figure 3).
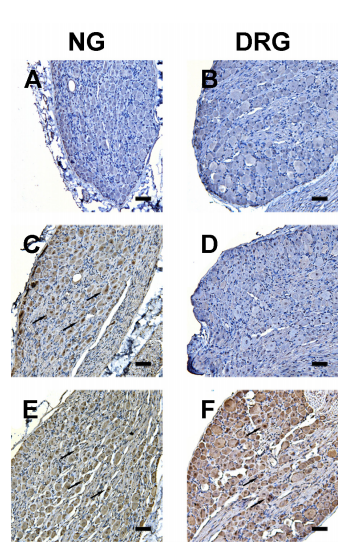
Figure 3. Representative photomicrographs showing c-Fos expression in nodose ganglion (NG) or T6- T10 dorsal root ganglion (DRG, corresponding to the duodenum) neurons. Fos immunoreactivity was not evident in NG or DRG neurons from rats receiving saline (luminally applied) treatment (A, B). Luminal application of progesterone (3.2 μmol) led to a marked increase in Fos- immunoreactive (IR) neurons (arrows) in the NG (C) but not the DRG (D) At the same dose, sc injected progesterone resulted in an increased number of Fos-IR neurons in both the NG (E) and DRG (F) Scale bar = 100 µm.
Progesterone effect depends upon vagal afferents
Luminal infusion of progesterone significantly decreased the VMR in rats undergoing perivagal vehicle pretreatment [F(1,24) = 26.2, vs. baseline, P < 0.01] (Figure 4A). Pretreatment of perivagal capsaicin markedly increased the baseline VMR, and also abrogated the analgesic activity of luminal progesterone [F(1,30) = 0.02, vs. baseline, P = 0.877] (Figure 4B).
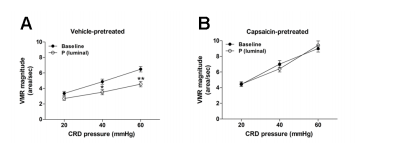
Figure 4. The antinociceptive effect of progesterone is dependent upon vagal afferents. (A) VMR before and after luminal administration of progesterone in rats subjected to perivagal vehicle (10% Tween 80 in olive oil) pretreatment (n = 5). (B) VMR before and after luminal administration of progesterone in rats subjected to perivagal capsaicin pretreatment (n = 6). *P < 0.05, **P < 0.01 vs. baseline (two-way repeated measures ANOVA)
The 5-HT/5-HT3R pathway mediates the effect of pro[1]gesterone
The average 5-HT concentration in the perfusate, was markedly increased compared to the vehicle[1]treated rats (P < 0.05) (Figure 5A), suggesting that progesterone, whether systemically or luminally administered, evokes 5-HT secretion into the duodenal lumen. Furthermore, pretreatment with luminal administration of the 5-HT3R antagonist granisetron obscured the attenuation of the VMR following luminal infusion of progesterone [F(1,30) = 2.2, vs. baseline, P = 0.148] (Figure 5B). Granisetron itself had no effect on the VMR (data not shown).
Figure 5. The antinociceptive effect of progesterone is mediated via the 5-HT/5-HT3R pathway. (A) 5-HT concentration in the duodenal perfusate from rats receiving luminal or sc administration of progesterone (n = 6). *P < 0.05 vs. vehicle (Student’s t-test). (B) The 5-HT3R antagonist granisetron (Gran) abrogated the antinociceptive effect of luminally applied progesterone. Granisetron (250 nmol) was luminally administered 20 min prior to progesterone (n = 6).
Progesterone effects are dependent upon IMMCs
Pretreatment with doxantrazole abrogated the antinociceptive effect of luminally applied progesterone [F(2, 45) = 13.5, P < 0.01] (Figure 6A). In line with the pain behavioral results, doxantrazole pretreatment blocked progesterone (luminally or sc administered) -evoked 5-HT secretion [luminal progesterone, F(2, 12) = 4.4, vs. DMSO + saline or doxantrazole + luminal progesterone, P = 0.04; sc progesterone, F(2, 12) = 5.7, vs. DMSO + saline or doxantrazole + sc progesterone, P = 0.018] (Figure 6B). DOX alone had no effect on the VMR magnitude or 5-HT content in the duodenal perfusate (data not shown). As Figure 6C shows, IMMCs from rats receiving saline treatment were mostly filled with dense granules and showed no evidence of activation. In the rats treated with an sc injection of progesterone, IMMCs showed morphological evidence of piecemeal degranulation, which was characterized by the formation of partially emptied granule containers. These containers were large and had focal deposits close to the granule membrane.
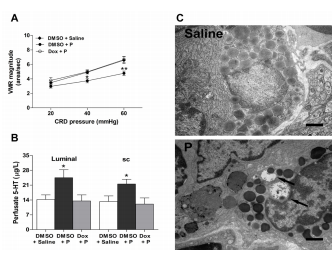
Figure 6. Intestinal mucosal mast cells (IMMCs) are involved in the effects of progesterone. (A) The IMMC stabilizer doxantrazole (dox) abrogated the antinociceptive effect of luminally applied progesterone (n = 6). *P < 0.05, **P < 0.01 vs. DMSO + saline or dox + P (two-way repeated measures ANOVA). (B) 5-HT secretion following luminally applied or sc injection of progesterone was inhibited by doxantrazole (n = 5) pretreatment. *P < 0.05 vs. DMSO + saline or dox + P (one-way ANOVA). (C) Representative electron micrographs showing piecemeal degranulation of intestinal mucosal mast cells from rats treated with P. A resting mast cell in the duodenal mucosa from a saline-treated rat with numerous homogeneous dense granules (top panel). Examples of mast cells undergoing piecemeal degranulation from a rat that received sc injection of progesterone (lower panel). Note that some granules have partially emptied their containers of dense contents, leaving large containers that often have focal dense deposits close to the granule membrane (arrows). Scale bar = 1 µm
Because mucosal enterochromaffin (EC) cells are the primary source of 5-HT secreted into the gut, we detected the content of chromogranin A, a marker of EC cell-derived 5-HT release[41]. We found that progesterone did not elicit obvious changes in the chromogranin A content in the serum or perfusate (Figure 7).
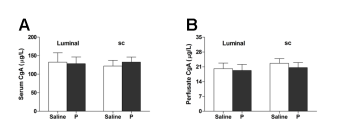
Figure 7. Progesterone (luminally or sc administered) had no effect on chromogranin A (CgA) content in serum (A, n = 6) or duodenal perfusate (B, n = 6).
The histamine content in the duodenal perfusate from progesterone-treated rats showed no obvious changes compared to the vehicle group (Figure 8A). Moreover, pyrilamine (a selective H1 antagonist) pretreatment did not affect the attenuation of VMR following luminal application of progesterone [F(1, 30) = 28.9, P < 0.01 vs. baseline] (Figure 8B)
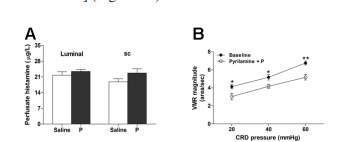
Figure 8. Histamine/H1 pathway is not involved in the antinociceptive effect of progesterone. (A) Histamine content in the duodenal perfusate from rats treated with progesterone (luminally or sc administered; n = 6). (B) Pyrilamine pretreatment did not influence the antinociceptive effect of luminally applied progesterone (n = 6). Pyrilamine (5 mg/kg) was ip injected 20 min prior to progesterone. *P < 0.05, **P < 0.01 vs. baseline (two-way repeated-measures ANOVA).
Co-administration of progesterone and estradiol exerts a synergetic effect The reduction in VMR magnitude in rats treated with luminal administration of progesterone in combination with estradiol was more dramatic than in rats treated with progesterone or estradiol alone [F(2,63) = 4.0, P= 0.024] (Figure 9A). In parallel, luminally applied progesterone plus estradiol induced more robust 5-HT secretion than progesterone or estradiol alone [F(2, 12) = 5.4, P = 0.033] (Figure 9B).
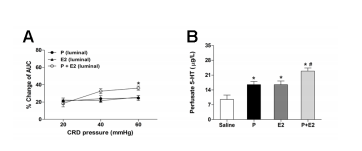
Figure 9. Co-administration of progesterone and estradiol exerts a synergetic effect. (A) The percentage of AUC change relative to baseline produced by luminal administration of progesterone in combination with estradiol (n = 8). *P < 0.05 vs. progesterone or estradiol alone (two-way repeated measures ANOVA). (B) Effect of luminal application of progesterone in combination with estradiol on duodenal 5-HT secretion (n = 5). *P < 0.05 vs. saline (Student’s t-test); # P < 0.05 vs. progesterone or estradiol alone (one way ANOVA).
Discussion
In the present study, we have demonstrated that progesterone attenuates visceral nociception through the vagal pathway, which is usually masked by its spinally mediated pronociception. Moreover, this vagally driven antinociception may involve IMMC[1]derived 5-HT and 5-HT3R signaling. Interestingly, progesterone and estrogen show a synergistic effect in the attenuation of the CRD response, suggesting that these two female hormones act synergistically in the regulation of visceral pain.
We used the administration route of luminal infusion, which has been demonstrated to preferentially act through the vagal pathway[23, 28], to observe the vagus[1]mediated effect of progesterone on visceral nociception. We found that luminally applied progesterone rapidly (within approximately 30 min) attenuates the VMR induced by CRD. The plasma progesterone level was not obviously changed, suggesting that this antinociception involves local rather than systemic action. The finding that luminally applied progesterone induced c-Fos expression (a cellular marker of neuronal activity) in nodose (but not DRG) neurons[18, 19, 22], along with the fact that progesterone failed to attenuate the VMR in rats pretreated with perivagal capsaicin, further supports the notion that progesterone-induced antinociception is associated with vagal (but not spinal) activation. As the vagus nerve does not innervate the lower gastrointestinal (GI) tract, this antinociception is likely to be exerted by an enhancement of vagally triggered descending inhibition of spinal pain processing; alternatively, it could be produced at the level of the brainstem (because the VMR depends on a spino-bulbo-spinal loop).
It has been reported that elevated levels of progesterone in the amygdala heighten the responsiveness to CRD[32]. In the present study we found that sc injected progesterone increased the VMR magnitude and c-Fos expression in T6-T10 DRG neurons, indicating a spinally mediated effect. We noticed that systemically injected estrogen also increased c-Fos expression in nodose ganglion neurons, indicating that besides acting through spinal mechanisms, systemic progesterone can also activate vagal afferents and produce antinociception, although this effect is relatively weaker and is usually masked by spinally mediated pronociception.
The actions of progesterone are mediated by nongenomic as well as genomic mechanisms[33, 34]. We found that the nuclear PR antagonist RU486 did not obviously influence the antinociceptive effect of progesterone, suggesting that the effect of progesterone may be mediated by a nongenomic mechanism
It has been demonstrated that progesterone is capable of stimulating selective mast cell secretion of 5-HT[20]. IMMCs are in close association with the vagal afferents that innervate the small intestinal mucosa and can functionally communicate with each other[21-22, 35]. In the current study we observed a rapid and significant increase in 5-HT (but not histamine) concentration in the duodenal perfusate after luminal or systemic administration of progesterone, which was abrogated by doxantrazole. Consistently, ultrastructural analysis showed that progesterone triggered piecemeal rather than anaphylactic degranulation of IMMCs, which is common in human IMMCs[36, 37]. These results, similar to those of previous studies[20], suggest that progesterone can elicit selective 5-HT secretion from IMMCs. Furthermore, we found that the antinociceptive effect of progesterone was inhibited by the 5-HT3R antagonist granisetron (but not the H1 antagonist pyrilamine). Given our previous findings that luminal 5-HT triggers vagally mediated antinociception via 5-HT3Rs[28], we infer that IMMC derived 5-HT may be involved in the antinociception effect of progesterone through the 5-HT3R pathway.
EC cells in the gut can also release 5-HT upon stimulation with nutrients, increased intraluminal pressure or mechanical stimuli[38-40], characterized by chromogranin A release[41, 42]. We found that chromogranin A concentration was not influenced by luminal or sc administration of progesterone, suggesting that the 5-HT secretion triggered by progesterone does not come from EC cells
We have previously reported that, like progesterone, estradiol inhibits visceral pain through the vagal pathway, which is mediated through IMMC-derived 5-HT secretion[23]. Therefore, we next investigated the effect of progesterone and estradiol co-administration. We found that the reduction in the VMR magnitude as well as duodenal 5-HT release produced by luminal application of progesterone plus estradiol was more obvious compared to rats treated with progesterone or estradiol alone. These results suggest that progesterone and estradiol exert a synergistic effect in the regulation of visceral pain, which may rely on the synergistic promotion of 5-HT release.
The modification of visceral sensory function is thought to be the major trigger for the development of functional disorders of the GI tract[43]. Given that female IBS patients usually present comorbid autonomic dysfunction[44-47], and considering our present and previous findings that both progesterone and estradiol modulate pain perception through spinal and vagal pathways[23], further research should determine whether progesterone and estrogen withdrawal lead to an imbalance between vagally mediated antinociception and spinally mediated nociception, which accounts for the exacerbation of pain symptoms during menses or in premenopausal women with IBS or other chronic pain syndromes.
In conclusion, progesterone attenuates visceral pain through vagal afferents. This effect is mediated through the selective secretion of 5-HT from IMMCs and downstream 5-HT3R signaling. These results provide new insights into the sex hormone modulation of IBS related visceral sensitivity.
Funding
This study is financially supported by the National Natural Science Foundation of China (Grant Nos. 31171106, 81070302 and 81270463)
Conflicts of interest statement
None declared.
References
[1] Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. Journal of Neuroscience Research. 2017;95:500-508.
[2] Nasser SA, Afify EA. Sex differences in pain and opioid mediated antinociception: Modulatory role of gonadal hormones. Life Science. 2019;237:116926.
[3] Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience. 2012;13:859-866.
[4] Adeyemo M, Spiegel B, Chang L. Meta‐analysis: do irritable bowel syndrome symptoms vary between men and women? Alimentary Pharmacology & Therapeutics. 2010;32:738-755.
[5] Berkley KJ. Sex differences in pain. Behavioral and Brain Sciences 1997;20:371-80.
[6] Houghton L, Lea R, Jackson N, et al. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471-474.
[7] Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. The Journal of Neuroscience. 2003;23:3908-3915.
[8] Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152:1182-1191.
[9] Lacroix-Fralish M, Tawfik V, Nutile-McMenemy N, et al. Progesterone mediates gonadal hormone differences in tactile and thermal hypersensitivity following L5 nerve root ligation in female rats. Neuroscience. 2006;138:601-608.
[10] LaCroix-Fralish ML, Tawfik VL, DeLeo JA. The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain. 2005;114:71-80.
[11] Zafra MA, Molina F, Puerto A. Effects of perivagal administration of capsaicin on post-surgical food intake. Autonomic Neuroscience. 2003;107:37-44.
[12] Iwasaki H, Collins J, Saito Y, et al. Naloxone[1]sensitive, pregnancy-induced changes in behavioral responses to colorectal distention: pregnancy-induced analgesia to visceral stimulation. Anesthesiology. 1991;74:927-933.
[13] Ren K, Wei F, Dubner R, et al. Progesterone attenuates persistent inflammatory hyperalgesia in female rats: involvement of spinal NMDA receptor mechanisms. Brain Research.2000;865:272-277.
[14] Sander HW, Kream RM, Gintzler AR. Spinal dynorphin involvement in the analgesia of pregnancy: effects of intrathecal dynorphin antisera. European journal of pharmacology. 1989;159:205-209.
[15] Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51:i2-i5.
[16] Bradesi S, Lao L, McLean PG, et al. Dual role of 5-HT receptors in a rat model of delayed stress[1]induced visceral hyperalgesia. Pain. 2007;130:56- 65.
[17] Gschossmann J, Mayer E, Miller J, et al. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterology & Motility. 2002;14:403- 408.
[18] Spaziani R, Bayati A, Redmond K, et al. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterology & Motility. 2008;20:336- 342.
[19] Elsenbruch S, Orr WC. Diarrhea-and constipation[1]predominant IBS patients differ in postprandial autonomic and cortisol responses. The American Journal of Gastroenterology. 2001;96:460-466.
[20] Vliagoftis H, Dimitriadou V, Theoharides T. Progesterone triggers selective mast cell secretion of 5-hydroxytryptamine. International Archives of Allergy and Immunology. 1990;93:113-119.
[21] Stead RH, Colley EC, Wang B, et al. Vagal i n f l u e n c e s o v e r m a s t c e l l s . A u t o n o m i c Neuroscience. 2006;125:53-61.
[22] Williams R, Berthoud H-R, Stead R. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation 1997;4:266-270.
[23] Yan XJ, Feng CC, Liu Q, et al. Vagal afferents mediate antinociception of estrogen in a rat model of visceral pain: the involvement of intestinal mucosal mast cells and 5-hydroxytryptamine 3 signaling. The Journal of Pain. 2014;15:204-217.
[24] Zhou SY, Lu YX, Owyang C. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;294:G1158-G64.
[25] Blackshaw LA, Page AJ, Partosoedarso E. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience. 2000;96:407-416.
[26] Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117:433-442.
[27] Sandhi J, Singh JP, Kaur T, et al. Involvement of progesterone receptors in ascorbic acid–mediated protection against ischemia-reperfusion–induced acute kidney injury. Journal of Surgical Research. 2014;187:278-288.
[28] Zhang L, Dong X, Liu Z, et al. Luminal serotonin time‐dependently modulates vagal afferent driven antinociception in response to colorectal distention in rats. Neurogastroenterology & Motility. 2011;23:62-e6.
[29] Jiang W, Kreis ME, Eastwood C, et al. 5-HT< sub> 3 and histamine H< sub> 1 receptors mediate afferent nerve sensitivity to intestinal anaphylaxis in rats. Gastroenterology. 2000;119:1267-1275.
[30] Van den Wijngaard R, Klooker T, Welting O, et al. Essential role for TRPV1 in stress‐induced (mast cell‐dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterology & Motility. 2009;21:1107-e94.
[31] Juanola C, Giralt M, Jiménez M, et al. Mucosal mast cells are involved in CCK disruption of MMC in the rat intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1998;275:G63-G7.
[32] Myers B, Schulkin J, Greenwood-Van Meerveld B. Sex steroids localized to the amygdala increase pain responses to visceral stimulation in rats. The Journal of Pain. 2011;12:486-494.
[33] Boonyaratanakornkit V, Edwards P. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25(3):139- 153.
[34] Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. European Journal of Endocrinology. 2003;148:281-292.
[35] Yu S, Kollarik M, Ouyang A, et al. Mast cell[1]mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007;293:G850-G6.
[36] Dvorak A, McLeod R, Onderdonk A, et al. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. International Archives of Allergy and Immunology. 1992;99:74-83.
[37] Santos J, Yang P, Söderholm JD, et al. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630-636.
[38] Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochemistry and Cell Biology. 1997;108:105- 113.
[39] Kellum JM, Albuquerque FC, Stoner MC, et al. Stroking human jejunal mucosa induces 5-HT release and Cl− secretion via afferent neurons and 5-HT4receptors. American Journal of Physiology. 1999;277(3):G515-20.
[40] Raybould HE, Glatzle J, Robin C, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;284:G367-G72.
[41] Cubeddu L, O’Connor D, Hoffmann I, et al. Plasma chromogranin A marks emesis and serotonin release associated with dacarbazine and nitrogen mustard but not with cyclophosphamide[1]based chemotherapies. British Journal of Cancer. 1995;72:1033.
[42] Okumiya K, Fujimiya M. Immunoelectron microscopic study of the luminal release of chromogranin A from rat enterochromaffin cells. Histochemistry and Cell Biology. 1999;111:253- 257.
[43] Mayer E, Gebhart G. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271-293
[44] Heitkemper M, Burr RL, Jarrett M, et al. Evidence for autonomic nervous system imbalance in women with irritable bowel syndrome. Digestive Diseases and Sciences. 1998;43:2093-2098.
[45] Li Y, Wu X, Yao H, et al. Secretin activates vagal primary afferent neurons in the rat: evidence from electrophysiological and immunohistochemical studies. American Journal of Physiology[1]Gastrointestinal and Liver Physiology. 2005;289:G745-G52.
[46] Theoharides TC, Kops SK, Bondy PK, et al. Differential release of serotonin without comparable histamine under diverse conditions in the rat mast cell. Biochemical Pharmacology. 1985;34:1389-1398.
[47] Tousignant-Laflamme Y, Goffaux P, Bourgault P, et al. Different autonomic responses to experimental pain in IBS patients and healthy controls. Journal of Clinical Gastroenterology. 2006;40:814-820

